Common product properties of argon gas
I. Physical properties
Argon is the most abundant of the noble gas family consisting of helium, neon, argon, krypton, and xenon. The common characteristic of these gases is that they are the least chemically active. Argon is a colorless, odorless and tasteless gas. Its boiling point is 185.87 ℃.
II. Chemical properties
Argon is a monatomic, chemically inactive gas. It doesn't react with other elements or compounds. Although a few compounds of red and other noble gases have been reported to have been prepared, such research can be considered of only scientific interest. Every attempt to synthesize compounds into common types has failed for all practical applications. These efforts include treating argon with oxidizing agents and reducing agents. Argon is stable because all its electrons are perfectly paired and there are no bonding orbitals.
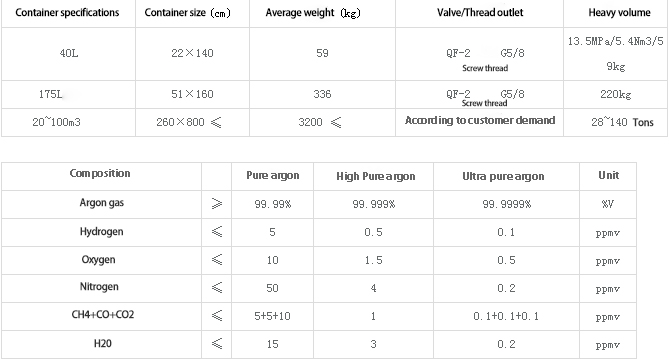
III. Technical specification standard of argon gas products:
Product specifications and technical indicators




 Facebook
Facebook YouTube
YouTube LinkedIn
LinkedIn Twitter
Twitter