Why does xenon work as an anaesthetic?
The relative strength receptors and acetylcholine receptors are only one of the explanations for the effects of nitrogen anesthesia.
1) Lipid theory:
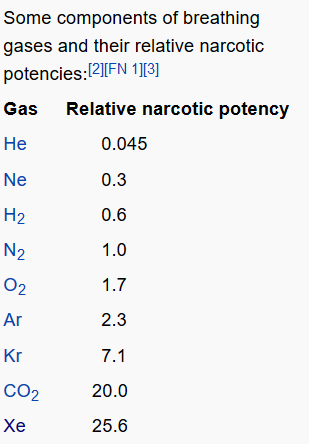
Lipid theory is actually a very classical explanation of the mechanism of anesthetics. The lipid theory holds that the central nervous system is rich in lipids, and according to the principle of similar compatibility, substances with a higher oil/water ratio are more easily absorbed by the central nervous system. If the partial pressure of a certain inert gas is very high, then the CNS cell membrane will absorb too much of this gas, which will lead to the increase of the cell membrane volume and the thickening of the cell membrane. Once the thickness is thicker than the ion channel, the ion permeability of the cell membrane will be dramatically affected and the membrane potential will change dramatically. In the case of a synapse, absorbing too much inert gas can alter the shape of the synapse, interfering with the release and reception of neurotransmitters, and acting as a narcotic. As can be seen from the above table, the xenon gas has a relatively high solubility in the lipid and therefore, according to the lipid theory, has the strongest anesthetic effect.
In fact, the same is true. Xenon has an anesthetic effect at normal pressure. Other inert gases, such as diving, require high-pressure environments to perform anesthetic functions. A classic example of the intoxicating effect of nitrogen is when diving, due to the high pressure under the water, the high pressure nitrogen can be dissolved in a large amount in the central nervous system, causing the anesthetic effect. At the same time, argon and krypton require lower pressure than nitrogen gas and are more anaesthetic.
This suggests that, to a certain extent, the lipid theory has its roots. On the other hand, if the pressure continues to increase, then mechanical action will dominate, will "press thin" the cell membrane again, exposing the ion channel, then the ion permeability will suddenly increase, so that the nerve excitability will increase, will cause another disease, the so-called HPNS -- high pressure nerve syndrome.
The relative strength of the anesthetic effects of common gases and nitrogen
2) Protein theory:
although lipid-like theory basically explains the principle of nitrogen intoxication effect, it cannot explain some specific symptoms of nitrogen intoxication and HPNS attack. Therefore, people are considering whether these inert gases can directly combine with some functional proteins on cells and exert effects. What the title says about inhibiting receptors on the cell membrane, that's what the protein theory is all about.
But there is actually no strong evidence for the protein theory, other than to say that the results from the available experimental data are very similar to the results of binding to the protein. For example, it is found that the relationship between partial pressure of gas and anesthetic effect is not linear, but a classic S-shaped curve. It is now accepted that inert gases act as antagonists of NMDA receptors and agonists of GABAA receptors, similar to nonpolar anesthetics (such as diethyl ether). But there's no real evidence that noble gases can actually bind to these receptors and work.
For xenon, the present research level, can only say that it is through "a variety of ways, including the antagonism of NMDA receptor, excited GABAA receptors, inhibit nChA and serotonin receptor 3 type a receptor, even restrain excited conduction or rely on the second messenger tool for the glutamate receptor inhibition of extracellular release of Ca2 + ions to anesthesia effect - but these have not yet been determined.




 Facebook
Facebook YouTube
YouTube LinkedIn
LinkedIn Twitter
Twitter