Separation Of Isotope Gases
The abundance of stable gas isotopes in nature, also known as the natural presence ratio, refers to the proportion of the isotope in all natural isotopes of this element. Abundance is generally expressed as a percentage. The abundance of artificial isotopes, such as krypton 85 (kr-85), which is widely used in HID lamps, is zero. Newradar gas isotopes of xenon of different abundance, imported xenon isotopes. Welcome to order.
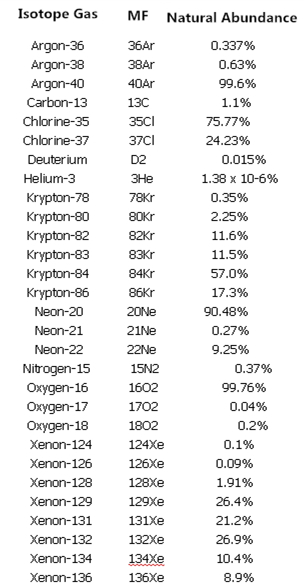
The atomic weights listed on the periodic table are actually the average of the various isotopes weighted by abundance, because isotopes tend to be evenly distributed in nature and the average value is more accurate. The following table:
The main separation methods of gas isotopes include gas diffusion method, ion exchange method, gas centrifugation method, distillation method, electrolysis method, electromagnetic method and current method, among which the gas diffusion method is the most mature. The use of enrichment involves isotope separation processes aimed at improving the abundance of specific isotopes of an element, such as the production of enriched uranium from natural uranium or heavy water from ordinary water.
Gas diffusion, the first commercially developed concentration method. The process relies on variations in the rate of movement of isotopes of different masses as they are converted into gaseous states. At each gas diffusion stage, when the high-pressure gas passes through the porous nickel film sequentially installed in the cascade, the gas of its light molecular gas passes faster through the porous membrane wall. This pumping process consumes a lot of power. Gases that have passed through the membrane are then pumped to the next stage, while those that remain in the membrane are returned to a lower level for recycling. At each stage, the concentration ratio increased only slightly. Uranium -235 enrichment to reactor level requires over 1000 levels.
Gas centrifugation, in which the gas is compressed through a series of high-speed rotating cylinders, or centrifuges. Isotopic heavy molecular gases are more likely to be enriched near the cylinder wall than light molecular gases. The gas enriched near the axis is exported and transferred to another centrifuge for further separation. As the gas passes through a series of centrifuges, its isotopic molecules are enriched. Gas centrifugation requires much less electricity than gas diffusion, so it has been used in most new enrichment plants.
- Prev: None
- Next: International gas industry development peak BBS





 Facebook
Facebook YouTube
YouTube LinkedIn
LinkedIn Twitter
Twitter