Will it turn water into hydrogen?
A combination of water, the chemical formula H2O, hydrogen and oxygen. It is not difficult to separate hydrogen from water. However, the collection and storage of hydrogen has always been a technical challenge, which has inhibited the practical application of photolysis of water to produce hydrogen. Researchers at the University of Science and Technology of China (USTC) have solved this problem. Using first-principles calculations, they proposed the first design of a material system that combines photolytic water for hydrogen production and hydrogen storage. The solution has the advantages of low cost, versatility and safe hydrogen storage.
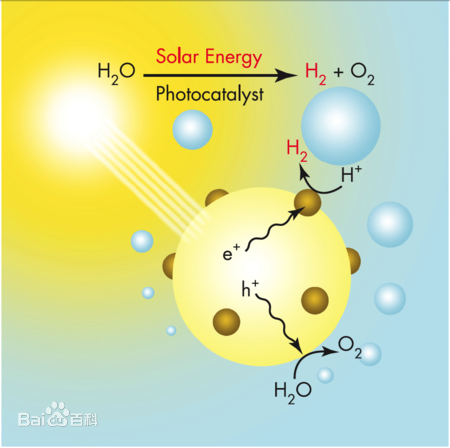
Photolysis of water to produce hydrogen stalled for a while
The seemingly perfect sustainable solution to a hydrogen economy was proposed back in the 1970s. Powered by inexhaustible sunlight, it splits water into hydrogen and oxygen.
"Hydrogen production depends on the migration of photoelectrons and holes to oxidation and reduction sites, respectively, so that the spacing between them must be smaller than the average electron free path, which is 10 -- 50nm. Such short spacing not only makes the reverse reaction inevitable, but also makes it difficult to separate and collect the hydrogen." On the other hand, storing hydrogen safely is a long-term challenge, University of Science and Technology of China researchers said. Hydrogen is extremely explosive and dangerous when mixed with oxygen. However, the commonly used high pressure liquefied metal hydrogen storage cost is high and inconvenient to use. Therefore, until solutions are developed to capture hydrogen at a low cost and store it safely, hydrogen production from solar photolysis water will not be able to be used effectively on a large scale.
Photolysis of water to produce hydrogen
Recent research achieves efficient purification of hydrogen
The researchers took their inspiration from the work of Nobel Prize-winning British scientist Sir Andre Gaim and Professor Heng 'an Wu of the University of Science and Technology of China. While graphene is capable of isolating all gases and liquids, it allows protons to pass freely. Taking advantage of this "convenient door" opened by nature to protons, Jiang Jun et al. designed a sandwich structure composed of two-dimensional carbon and nitrogen materials and graphene-based materials.
In this sandwich system, carbon and nitrogen materials are sandwiched between two layers of graphene modified with functional groups. In the process, graphene only releases hydrogen atoms, and the hydrogen produced by photolysis water cannot penetrate the graphene material. As a result, the hydrogen molecules produced by photolysis water will be safely retained in the sandwich composite system. At the same time, O2, OH and other systems can not enter the complex system, which inhibits the occurrence of reverse reaction, and achieves safe hydrogen storage under high hydrogen storage rate.




 Facebook
Facebook YouTube
YouTube LinkedIn
LinkedIn Twitter
Twitter