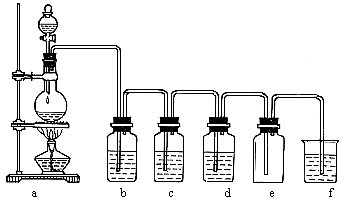
Concentrated hydrochloric acid distillation
Because hydrogen chloride and water form azeotropes, concentrated hydrochloric acid can only partially obtain hydrogen chloride gas during distillation, and at the same time produce a large amount of azeotropic dilute hydrochloric acid.
Therefore, dilute hydrochloric acid composed of azeotrope at the bottom of desorption tower is used as the absorption liquid of hydrogen chloride gas in this process. Part of the absorbed concentrated hydrochloric acid is used as the washing liquid of hydrogen chloride gas, and part is used as the raw material of desorption tower. The mixed acid produced by washing is sold as a commodity.
After being cooled by the air cooler, the gas (hydrogen chloride and impurities) from the reactor of the potassium sulfate unit first enters the cooling scrubber and scrubber tower (packed tower), and then enters the absorption tower (falling film tower) after being washed by concentrated hydrochloric acid to remove potassium chloride and sulfuric acid.
Distillation of concentrated hydrochloric acid produces HCL gas
Under normal pressure, dilute hydrochloric acid was used as the absorbent, and after two stages of absorption, concentrated hydrochloric acid was obtained at the bottom of the second stage falling film absorber. A small amount of unabsorbed hydrogen chloride and gas impurities, through the hydrogen chloride recovery tower and tail gas cleaning tower further absorption of hydrogen chloride with water, tail gas through the induced draft fan to empty. Control the sulfuric acid content in the bottom mixture of the last stage scrubber tower to be less than 10% (mass fraction), and send it into the mixed acid storage tank.
The concentrated hydrochloric acid at the bottom of the second stage falling film absorber is fed into desorption hydrochloride to desorb hydrogen chloride gas. The top temperature of the desorption tower is controlled at about 60℃, and the bottom temperature is controlled at about 120℃.
The outlet pressure of hydrogen chloride gas at the top of desorption tower is controlled at about 30kPa. The dilute hydrochloric acid at the bottom of the desorption tower is cooled by water and then returned to the top of the falling film absorption tower as the absorption liquid. After the hydrogen chloride gas at the top of desorption tower is cooled with water and refrigerated with -15℃ brine, the purity reaches more than 99.9% (mass fraction) and is sent to the next process.




 Facebook
Facebook YouTube
YouTube LinkedIn
LinkedIn Twitter
Twitter