Manufacturing chlorine gas
Technical history
Production methods for chlorine gas have undergone a long process of development. In 1774, Swedish chemist Scheele used pyrolusite (containing manganese dioxide) and concentrated hydrochloric acid, first made chlorine gas, its reaction equation is:
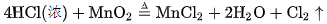
However, since hydrochloric acid could not be produced in large quantities at that time, this method was limited to the preparation of chlorine gas in the laboratory. Later, French chemist Bertolet put the mixture of sodium chloride, pyrolusite and concentrated sulfuric acid into the lead distiller, and got chlorine after heating. The reaction equation is as follows:
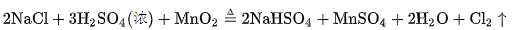
Because this method raw material is easy to get, so, from 1774 Scheler made chlorine to 1836, people have been using the method invented by Bertolei to produce chlorine.
1836 ancient sag developed a coking tower, used to absorb the way the cloth blue method in the production of soda ash Na2CO3 () to remove hydrogen chloride gas process (before this gas is considered to be a kind of waste gas containing hydrogen chloride, beginning from the ancient sag, just got the full use of) by hydrochloric acid, hydrochloric acid from now on become a cheaper acid, can be widely used. Scheele's method of producing chlorine gas was improved, and by this time it had become a method of producing chlorine gas on a large scale.
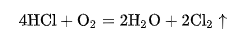
In 1868, Dickon and Honte invented the method of using copper chloride as catalyst to produce chlorine gas by oxidizing hydrogen chloride gas with oxygen in the air during heating. The reaction equation is as follows:
This method is called the Dickon method.
These methods of producing chlorine gas, although they have played a role in the history, but compared with the electrolysis method of producing chlorine gas, no matter from the economic efficiency or from the scale of production, are greatly inferior. When electrolysis became practical in production, the above methods of producing chlorine gas were gradually phased out.
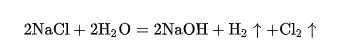
The birth of electrolysis dates back to 1833. After a series of experiments, Faraday found that chlorine gas could be obtained when an electric current was applied to an aqueous solution of sodium chloride. The reaction equation was as follows:
Later, the British scientist Watt also discovered this method, and in 1851 received a British patent for the production of chlorine gas. But because there were no practical DC generators to generate enough current, electrolysis remained on a laboratory scale rather than on an industrial scale. It was not until the 1870s and 1880s, when better DC generators appeared, that the electrolysis method was widely used. Since then, the industrial production of chlorine gas has entered a new era. However, at that time, the electricity used to produce chlorine by electrolysis was extremely mercury, resulting in a considerable amount of mercury vapor mixed in the chlorine and hydrogen obtained by electrolysis. This "mercury chlorine" is very harmful to the environment, so the new "ion exchange membrane method" to make chlorine gas, more environmental protection, more energy saving. (Chlorine from mercury is the dominant method of producing chlorine, with 46% of China's chlorine in 2010 and 50.1% of Western Europe's chlorine in 2000 being produced by this method)





 Facebook
Facebook YouTube
YouTube LinkedIn
LinkedIn Twitter
Twitter